Efficiency Loss in Solar Batteries: Causes and Solutions
Electrical to chemical conversion, also known as electrochemical conversion, involves the conversion of electrical energy into chemical energy through a redox reaction. This process is used in various applications, such as batteries, fuel cells, and electroplating.
During this conversion, there is a loss of energy in the form of heat. This loss is due to various factors, such as resistive losses in the electrical circuit, overpotential at the electrode surfaces, and inefficiencies in the electrochemical reaction itself.
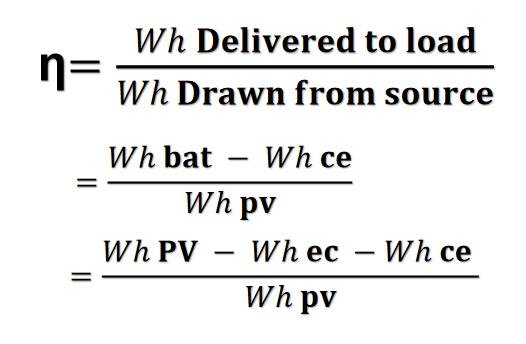
One of the main reasons for the loss is the overpotential at the electrode surfaces, which is the excess energy required to drive the electrochemical reaction. This energy is lost as heat and cannot be used to do work.
Another factor that contributes to the loss is the inefficiency of the electrochemical reaction. In most cases, the electrochemical reaction does not proceed with 100% efficiency, and there is some loss of energy in the form of heat.
Overall, the loss from electrical to chemical conversion is a result of the inherent inefficiencies in the electrochemical process and the resistive losses in the electrical circuit. While these losses cannot be completely eliminated, efforts are made to minimize them through the optimization of the electrode materials, electrolytes, and operating conditions.